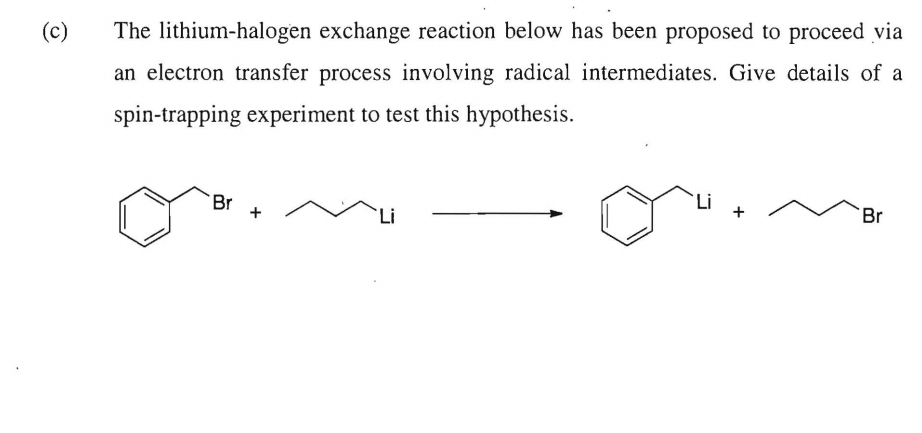
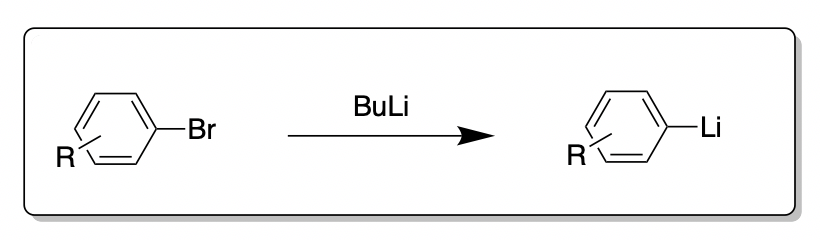
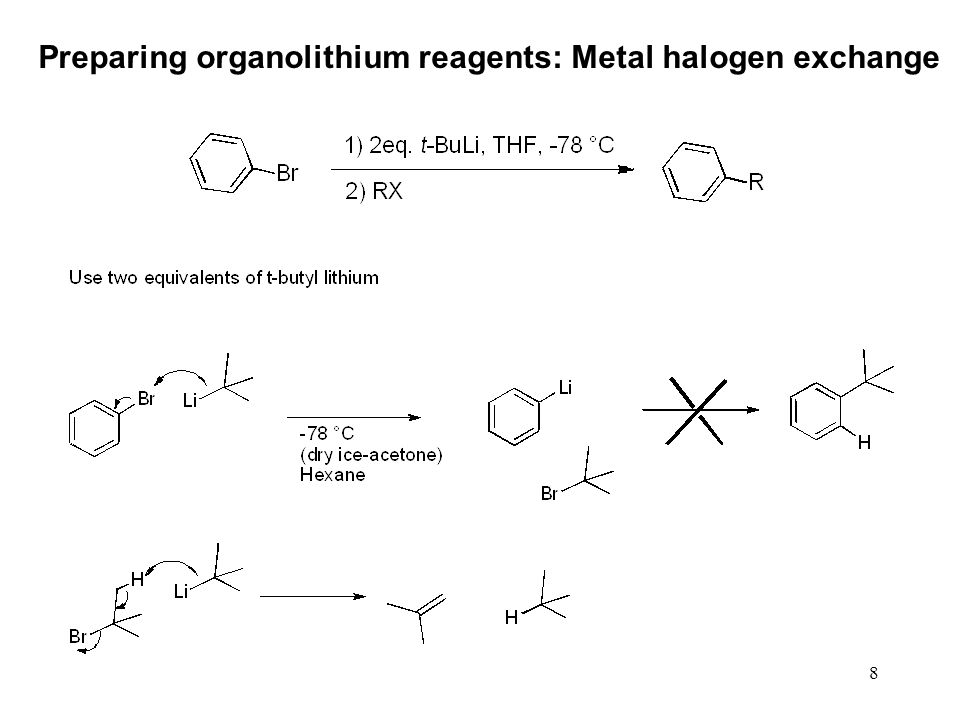
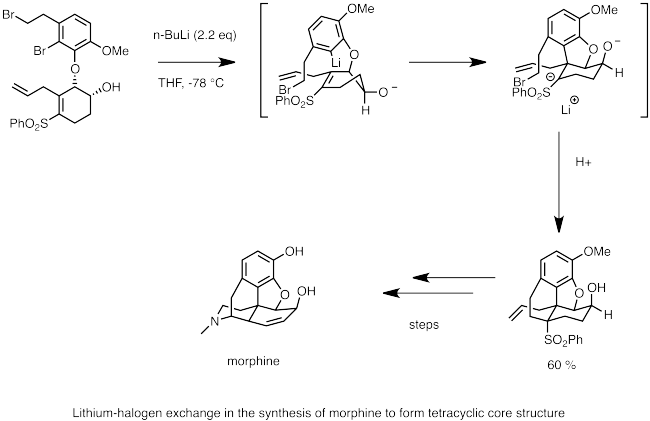
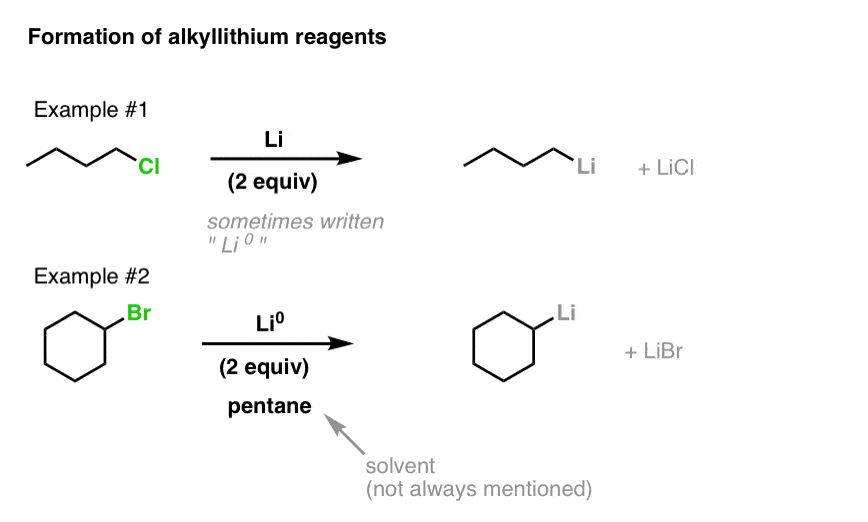
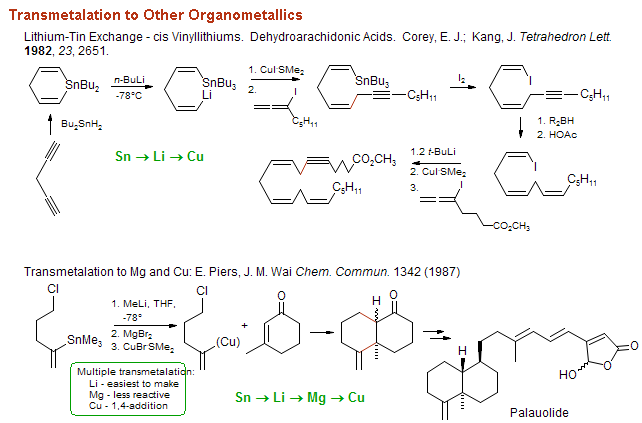
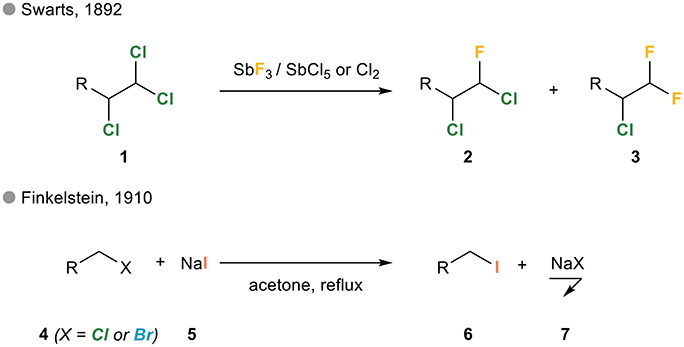
![The Mechanism of Lithium-Halogen ??The Mechanism of Lithium-Halogen Exchange ... Wittig restricted his research to ... ESR demonstrates radicals may be generated but it is not definitive - [Download PDF] The Mechanism of Lithium-Halogen ??The Mechanism of Lithium-Halogen Exchange ... Wittig restricted his research to ... ESR demonstrates radicals may be generated but it is not definitive - [Download PDF]](https://img.dokumen.tips/img/1200x630/reader018/reader/2020011121/5a861c6e7f8b9ad30c8cdde8/r-1.jpg?t=1636664691)
The Mechanism of Lithium-Halogen ??The Mechanism of Lithium-Halogen Exchange ... Wittig restricted his research to ... ESR demonstrates radicals may be generated but it is not definitive - [Download PDF]
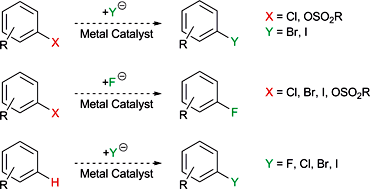
Metal-catalysed halogen exchange reactions of aryl halides - Organic & Biomolecular Chemistry (RSC Publishing)

Regioselective lithium–halogen exchange and palladium-catalyzed cross-coupling reactions of 2,4-dihaloquinolines - ScienceDirect
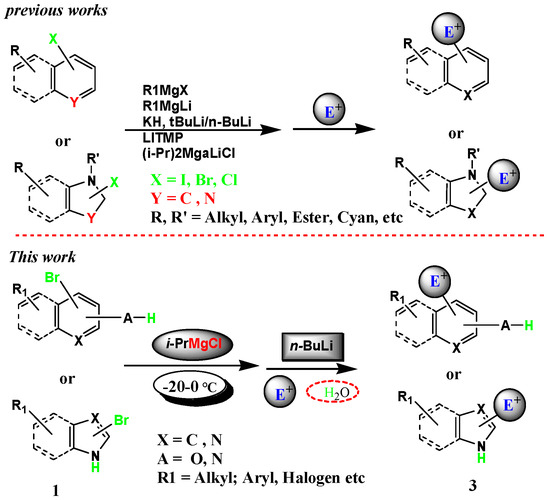
Molecules | Free Full-Text | Halogen–Metal Exchange on Bromoheterocyclics with Substituents Containing an Acidic Proton via Formation of a Magnesium Intermediate | HTML
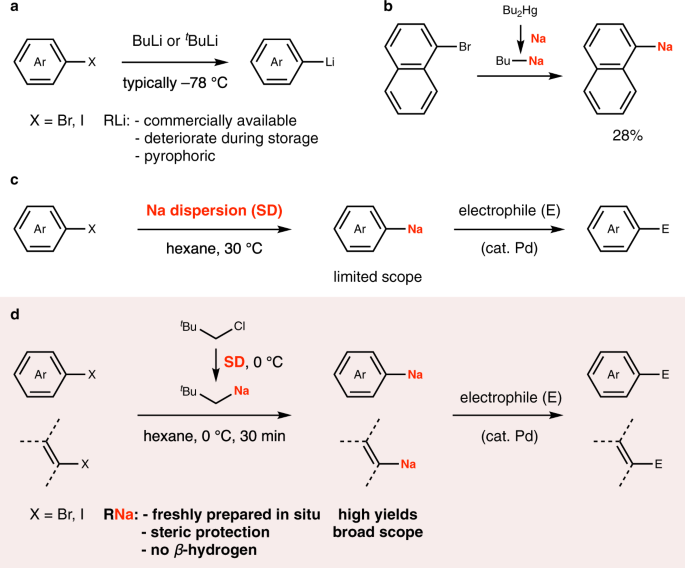
Halogen–sodium exchange enables efficient access to organosodium compounds | Communications Chemistry