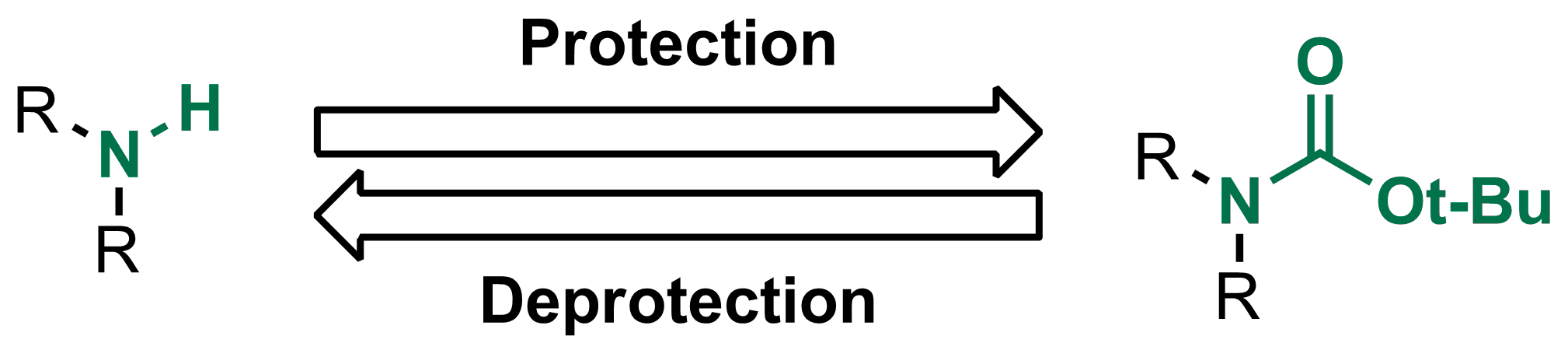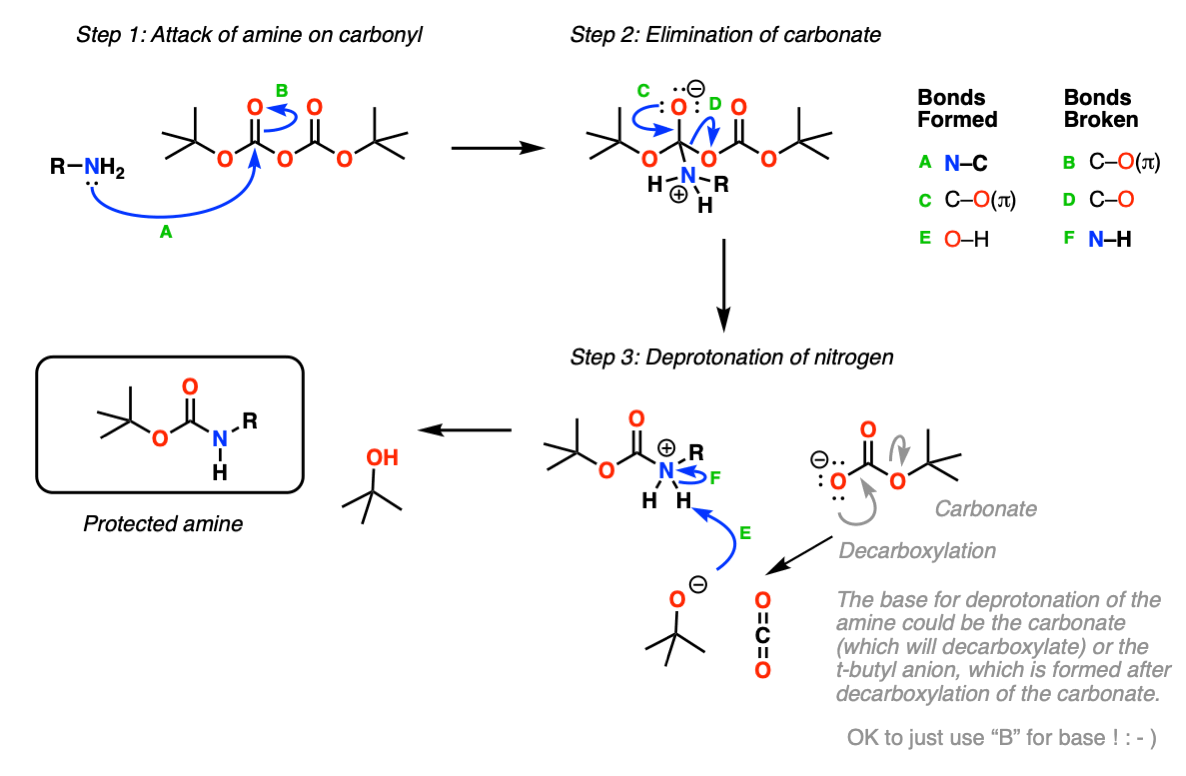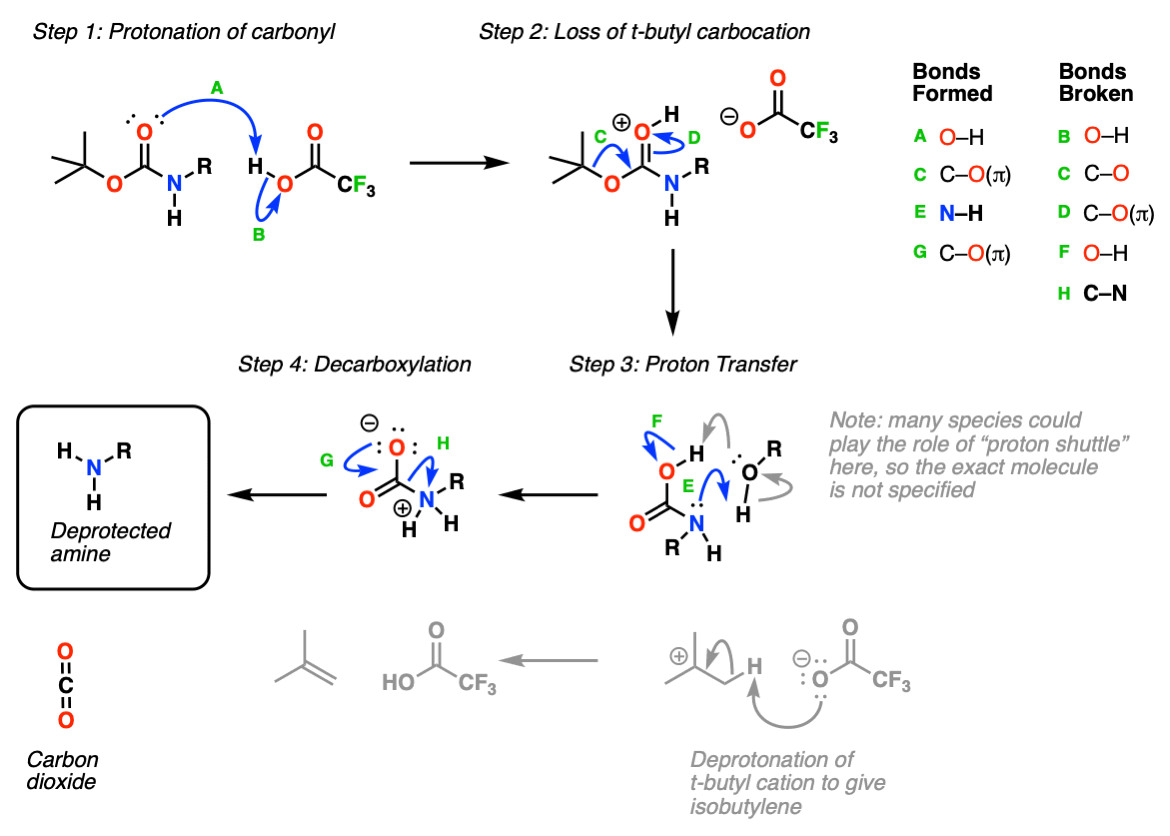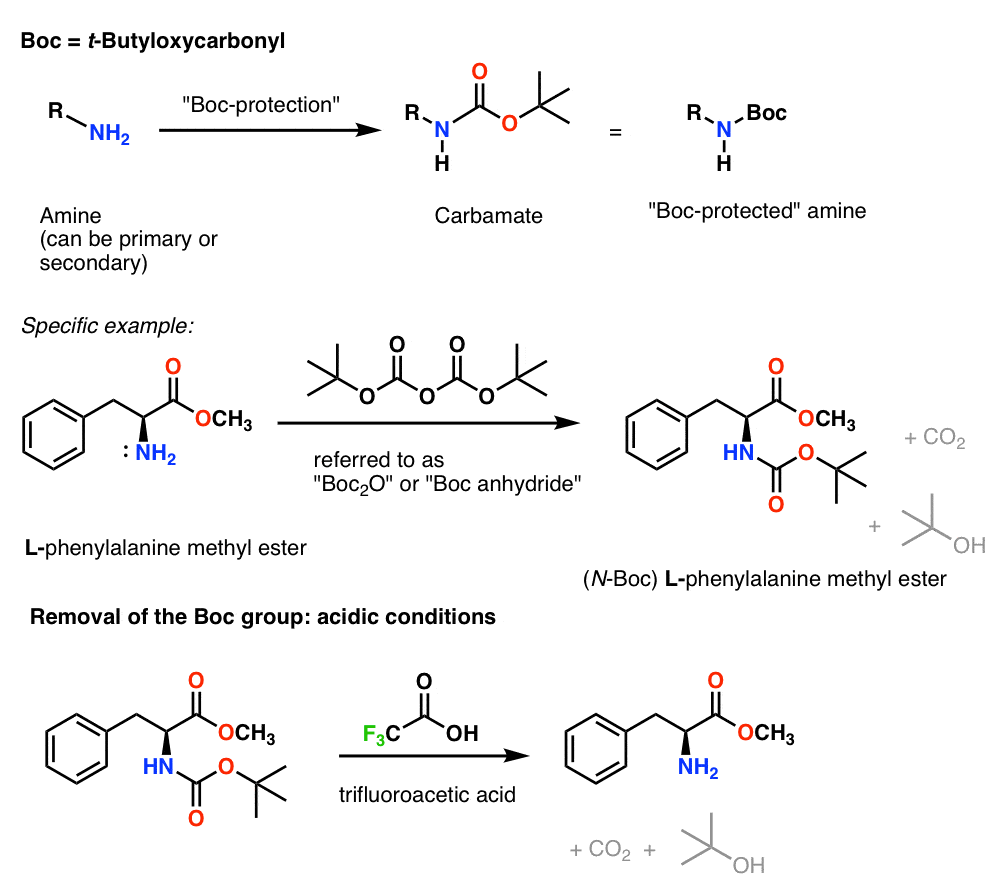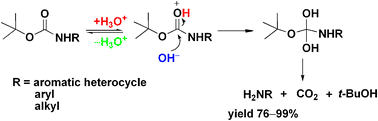
Boiling water-catalyzed neutral and selective N-Boc deprotection - Chemical Communications (RSC Publishing)
ChemSpider SyntheticPages | BOC deprotection of an aminophenylethyl methanesulfonate using hydrochloric acid

Mild deprotection of the <i>N</i>-<i>tert</i>-butyloxycarbonyl (<i>N</i>-Boc) group using oxalyl chloride. - Abstract - Europe PMC

organic chemistry - Mechanism for cyclic enamine formation after N-Boc deprotection - Chemistry Stack Exchange

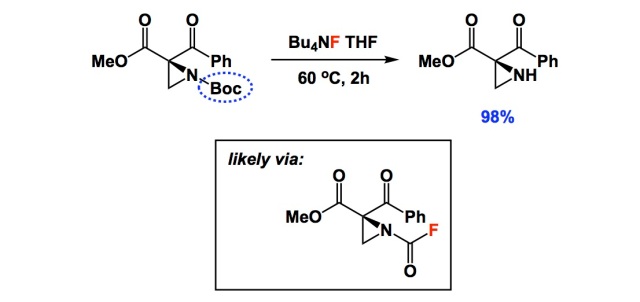
organic chemistry - Deprotection of Boc using TAF to obtained free amine group - Chemistry Stack Exchange

Suppression of Side Reactions During Final Deprotection Employing a Strong Acid in Boc Chemistry: Regeneration of Methionyl Residues from Their Sulfonium Salts | Semantic Scholar
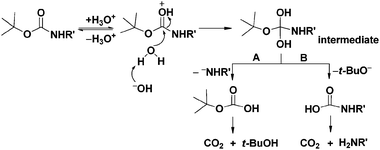
Boiling water-catalyzed neutral and selective N -Boc deprotection - Chemical Communications (RSC Publishing) DOI:10.1039/B910239F